Understanding Mycosis Fungoides: An Overview
Mycosis fungoides, a type of lymphoma, is a rare and unique condition that primarily affects the skin. Its rarity and uniqueness make the quest for effective treatment a challenge. It's characterized by patches, plaques, and tumors on the skin, which can sometimes progress to involve lymph nodes and internal organs. The disease is slow-progressing and can take many years to evolve, which makes it an intriguing and complex medical puzzle that researchers and physicians are working hard to solve.
The Significance of Clinical Trials in Medicine
Clinical trials play a crucial role in bridging the gap between scientific research and effective treatment options. They are the backbone of medical advancements, providing invaluable data on the safety and efficacy of new drugs, therapies, and procedures. Without clinical trials, we would not have many of the life-saving treatments we have today. They offer us a systematic and controlled method to test new treatments and to improve the standards of care.
Exploring Clinical Trials for Mycosis Fungoides
The treatment landscape for mycosis fungoides is continuously evolving, thanks to ongoing clinical trials. These trials are exploring new therapeutic options, including targeted therapies, immune checkpoint inhibitors, and CAR-T cell therapies, among others. The goal of these trials is not only to find a cure but also to improve the quality of life of patients living with this condition, by reducing symptoms and controlling the disease progression.
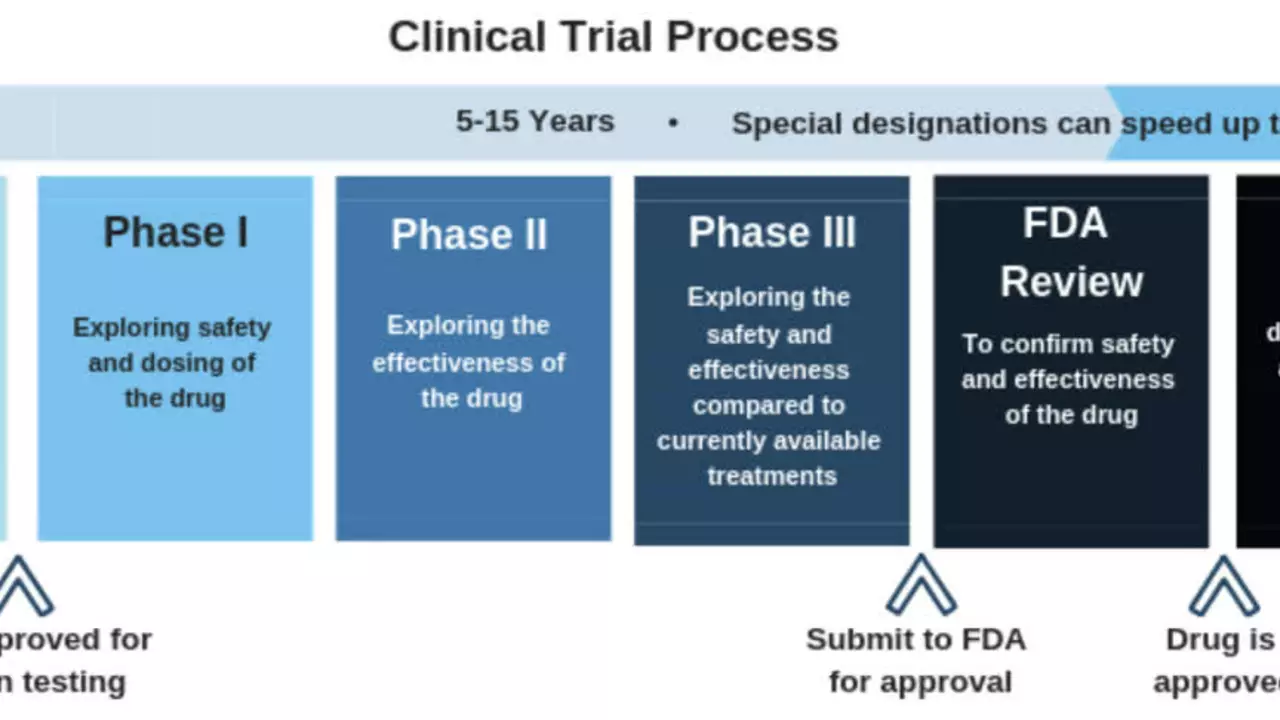
Understanding the Process of Clinical Trials
Clinical trials are a multi-step process. They typically start with pre-clinical testing in the lab, before moving on to phase I, II, and III trials, each with a specific objective and criteria. Phase IV trials, or post-marketing surveillance trials, follow after the treatment has received approval. Each step is crucial in assessing the safety, efficacy, side effects, and ideal dosage of the treatment being studied.
Participating in a Clinical Trial: What to Expect
If you or a loved one has been diagnosed with mycosis fungoides, participating in a clinical trial could be an option worth considering. It's crucial, however, to fully understand what participation entails. This includes understanding the potential risks and benefits, the trial design, the commitment required, and what to expect during and after the trial. It's also essential to discuss this with your healthcare team to make an informed decision.
Impact of Clinical Trials on the Future of Mycosis Fungoides Treatment
While mycosis fungoides remains a rare and complex disease, the future looks promising, largely due to clinical trials. The data gathered from these trials is not only advancing our understanding of the disease but also paving the way for more effective and personalized treatment options. Looking ahead, these trials are our best hope in finding a cure for this disease.
The Role of Patients and Healthcare Professionals in Advancing Clinical Trials
Last but not least, it's important to highlight the role of patients and healthcare professionals in advancing clinical trials. Their participation is critical in moving research forward. Patients bring invaluable first-hand experience of living with the disease, while healthcare professionals bring their expertise and commitment to patient care. Together, they contribute significantly to the progress being made in the treatment of mycosis fungoides.